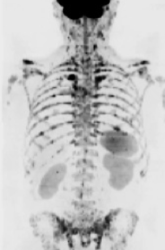
Acute kidney injury: prevention, detection and management
This NICE guideline provides recommendations for non-specialists for the identification and referral guidance for children and adults who present with symptoms or risk factors of acute kidney injury.