Driving diagnosis: Education and outreach
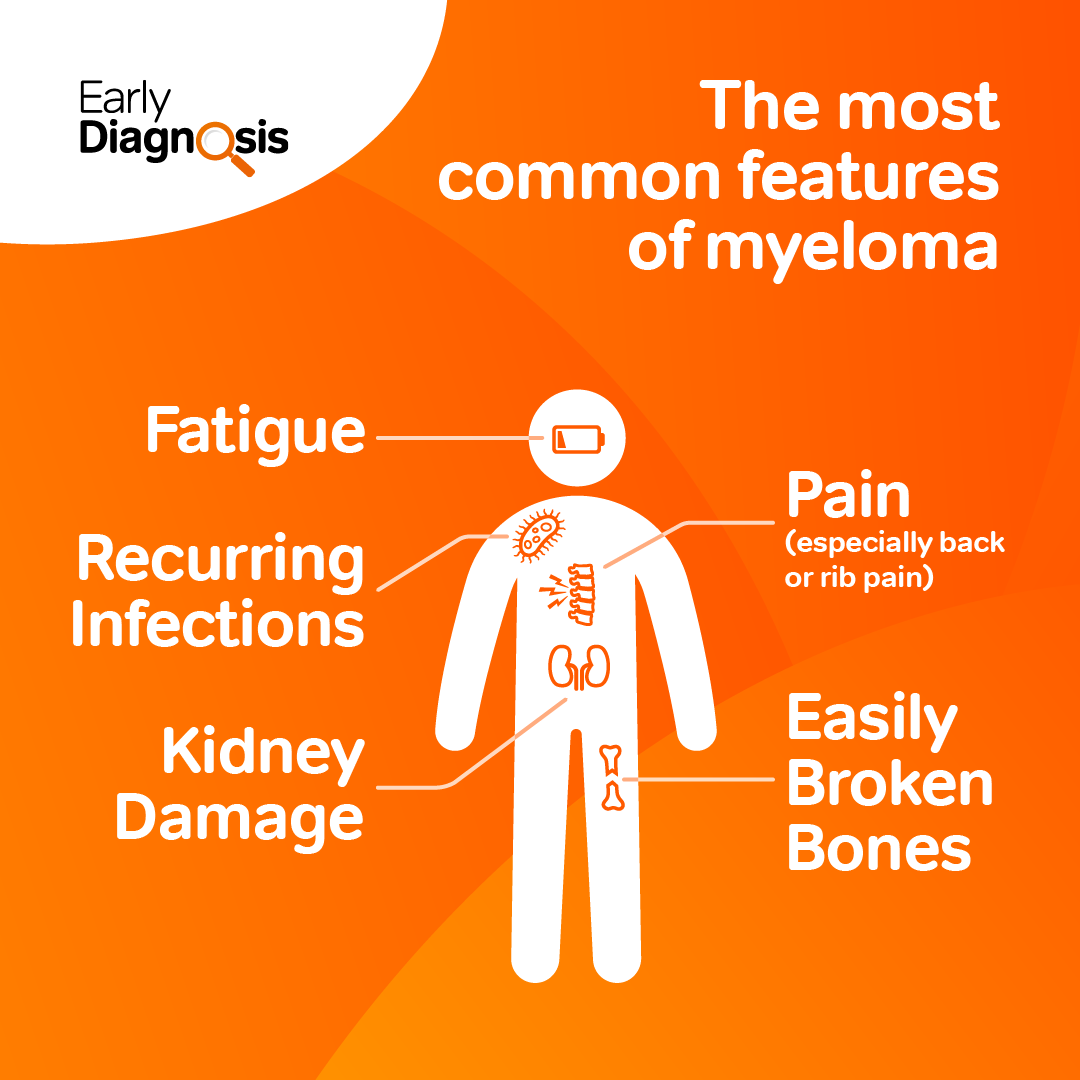
Our Clinical Practice Services Team continued their focus on raising awareness of myeloma in primary care. This included launching a guide for GPs and highlighting the importance of early diagnosis at national conferences.
Details