The following questionnaire can determine if you are at risk of developing obstructive sleep apnea (OSA) by screening for 4 self-reportable and 4 demographic characteristics. Note this is Property of the University Health Network. Modified from Chung F et al. Anesthesiology 2008; 108: 812-821, Chung F et al Br J Anaesth 2012; 108: 768-775, Chung…
The haematological malignancies patient report outcomes (HM-PRO) measure has been developed to support patients and their clinicians in discussing, addressing and better recording quality of life issues that matter patients. Access the questionnaire here.
Common Terminology Criteria for Adverse Events (CTCAE) is widely accepted as the standard classification and severity grading scale for adverse events in cancer therapy, clinical trials and other oncology settings. Version 5.0 is the most updated document (November 27, 2017)
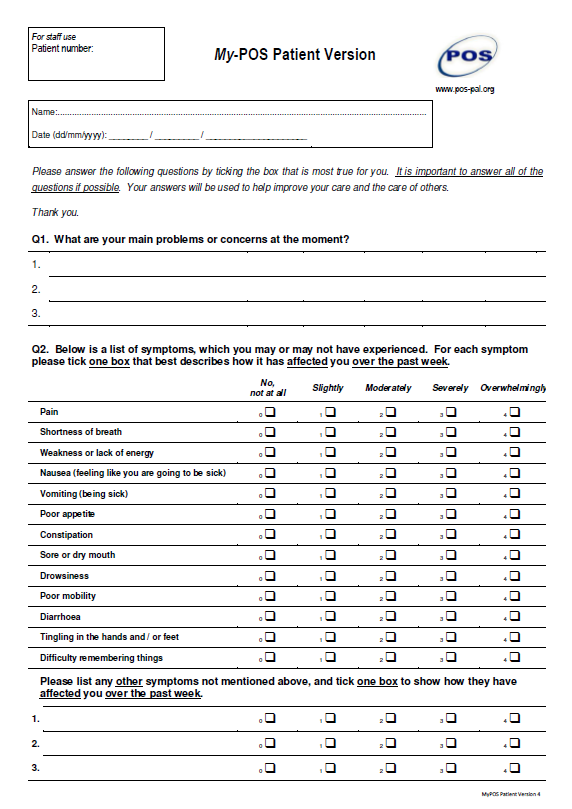
MyPOS is a myeloma-specific version of the Palliative care Outcome Scale recently developed and validated to measure myeloma-specific quality of life issues and intended for use in clinical care. Click here to register and view
European Organisation for Research and Treatment of Cancer (EORTC) assessment of quality of life of myeloma patients.
European Organisation for Research and Treatment of Cancer (EORTC) assessment of quality of life of cancer patients involving a 30 item questionnaire.
The British Pain Society has provided a pain rating scale to assist with the assessment of pain.
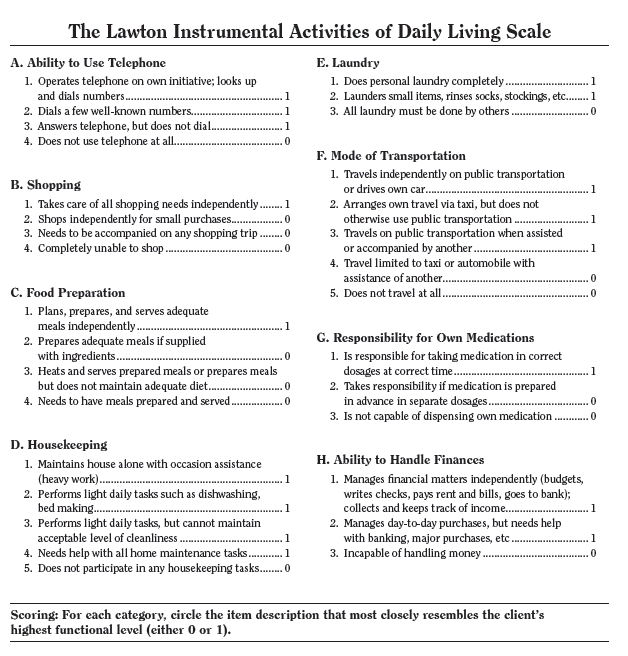
The Lawton Instrumental Activities of Daily Living (IADL) scale assesses the more complex activities of daily living necessary for living in the community, such as shopping, cooking and managing finances. These skills are usually lost before other activities of daily living functions are, such as bathing, dressing and eating.