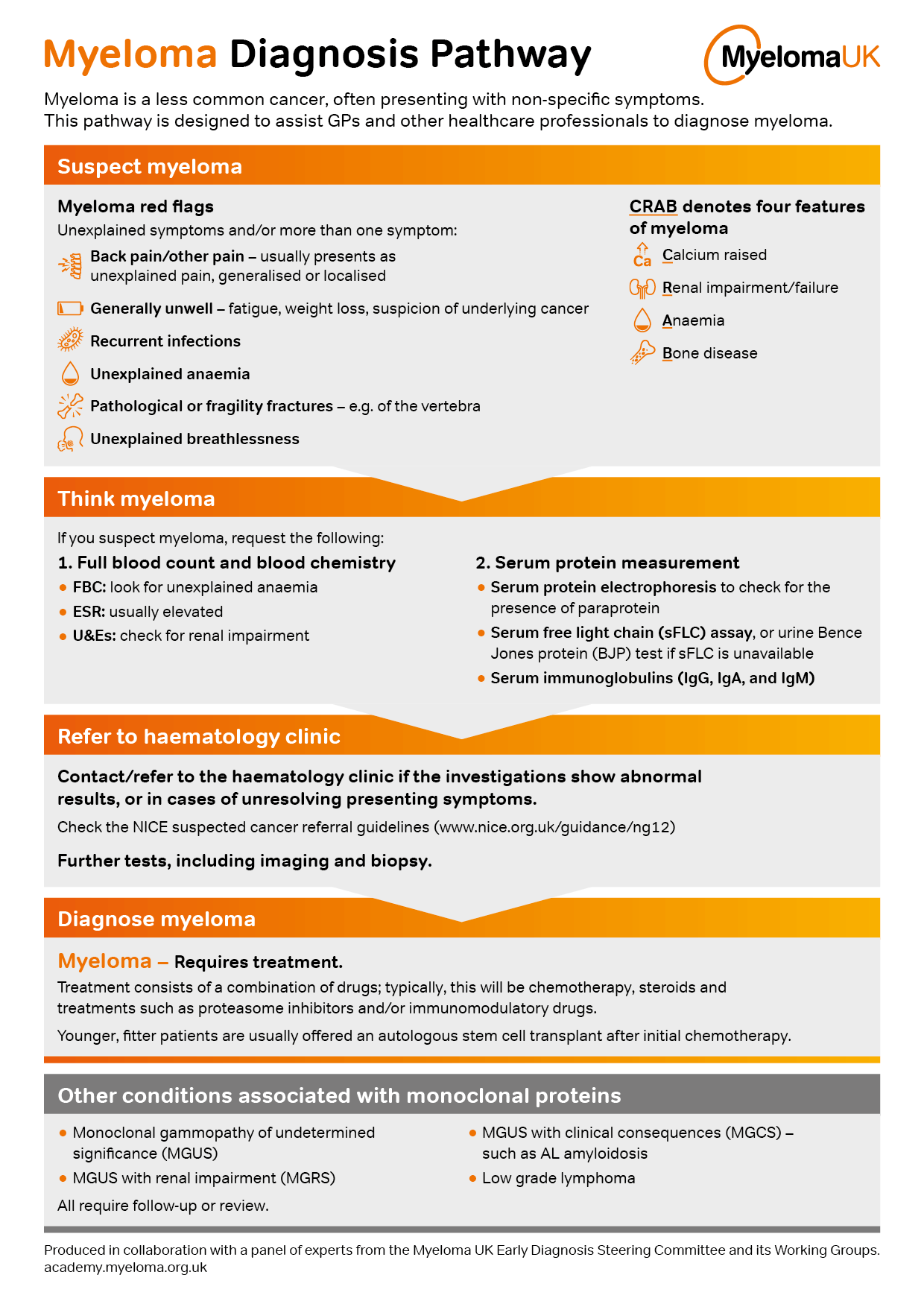
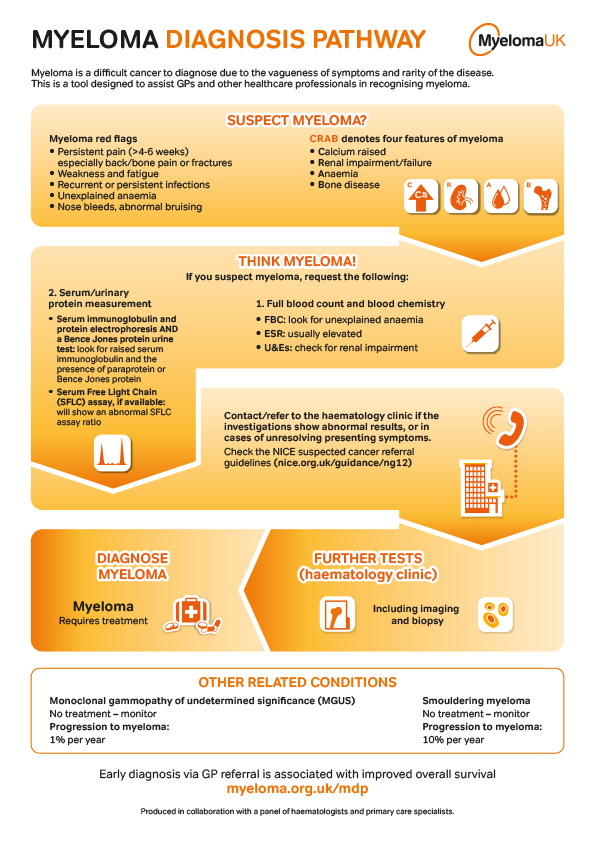
Myeloma UK and the UK Myeloma Research Alliance launch new research programme
Myeloma UK is launching a new research programme to continue support for patient-centric myeloma clinical trials in the UK, the UK Myeloma Research Alliance-Myeloma UK-Concept and Access Research Programme (UKMRA-Myeloma UK-CARP). This initiative is part of an ambitious new strategy Myeloma UK recently published with a mission to make myeloma history. One of our key…
Details